Learning with McCell - part 3: Karl Fischer Method
Ready for a new lesson with McCell? Today he will tell us about the Karl Fischer Method.
Definition / Explanation:
This method is used for the quantitative determination of water with a titration. The volume of water ranges from a few ppm up to the saturation level.
Scope of application:
This method has a large spectrum of use and is for example used to determine the water volume in food (e.g. sweets), in chemicals, medicines and cosmetics. At Mibelle Biochemistry it is used to determine the volume of water in alcohols, oils and powders.
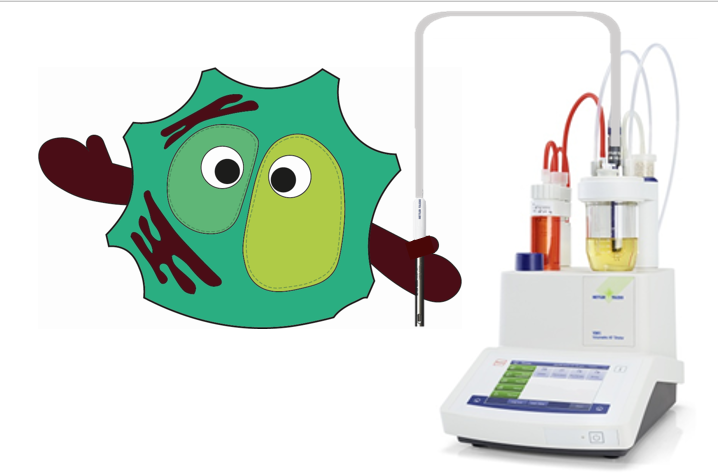
Mode of operation:
The titrator must first be switched on to make a zero adjustment.
For the factor, which is involved in the calculation, a 100% water sample is used. The sample is then weighed in based on the expected water content. In the vessel there is a conductivity electrode, which is performing the measurement. The water in the sample forms a complex with the iodine in the titration solution. This complex is giving a conductivity, which then can be measured. The water content is determined from the titrator based on the conductivity, the needed volume of the titration solution and the weighted sample. A determination is always made in triplicates to ensure that the measurements are correct. At the end of all measurements, pure water is measured to check whether the measurements are correct or not. If the value does not reach 99.5 to 100% of water, then all measurements must be repeated. The entire assay with factor, measurements and verification usually takes an hour per sample.








